

- #Periodic table of elements chemistry reference table how to#
- #Periodic table of elements chemistry reference table download#
How important is Mendeleev’s periodic table? You are also invited to visit the interactive version of the periodic table, available at PCC Group’s Chemical Academy.
#Periodic table of elements chemistry reference table download#
We encourage you to download one of the latest versions of the periodic table of elements (PDF, educational Mendeleev’s table for printout), which has been prepared by PCC Group experts. In some versions of the periodic table of elements indicating the valence, we can find even more information referring to particular properties, such as the boiling and melting points. So what is a period in the periodic table? The numbers of periods express the quantity of atomic electron shells (the fewer shells, the simpler the atomic structure of the element).īased on the system designed, each individual field in the table of elements may contain the following information about the element: The group numbers tell us how many valence electrons the elements have. The horizontal lines are called periods, while the columns are defined as groups.
#Periodic table of elements chemistry reference table how to#
Since we already know the basics, let’s answer the following question: where is the group and where is the period? Besides, what do the data placed by each symbol indicate? In short, how to read the periodic table? The periodic table consists of groups and periods that contain elements, and the position of these elements translates into their particular characteristics. If we take a closer look at the table, the information it contains should be easy to read even by a layperson. How to read the periodic table of elements? Mendeleev’s table also includes non-metallic gases and liquids, examples being nitrogen, chlorine, and iodine. It indicates that the vast majority of elements are metals (including precious metals) having the form of solids, such as copper, zinc, magnesium or gold. When we search for the periodic table of chemical elements for printout, we can also find a simplified diagram that only covers metals and non-metals. Mendeleev’s periodic table: division into metals and non-metals

The periodic table also contains elements that are still subject to research and are classified into the group of elements with unknown properties (these include, for example, the transuranic Moscovium and the synthetic Oganesson that does not exist as a natural, earthly deposit).
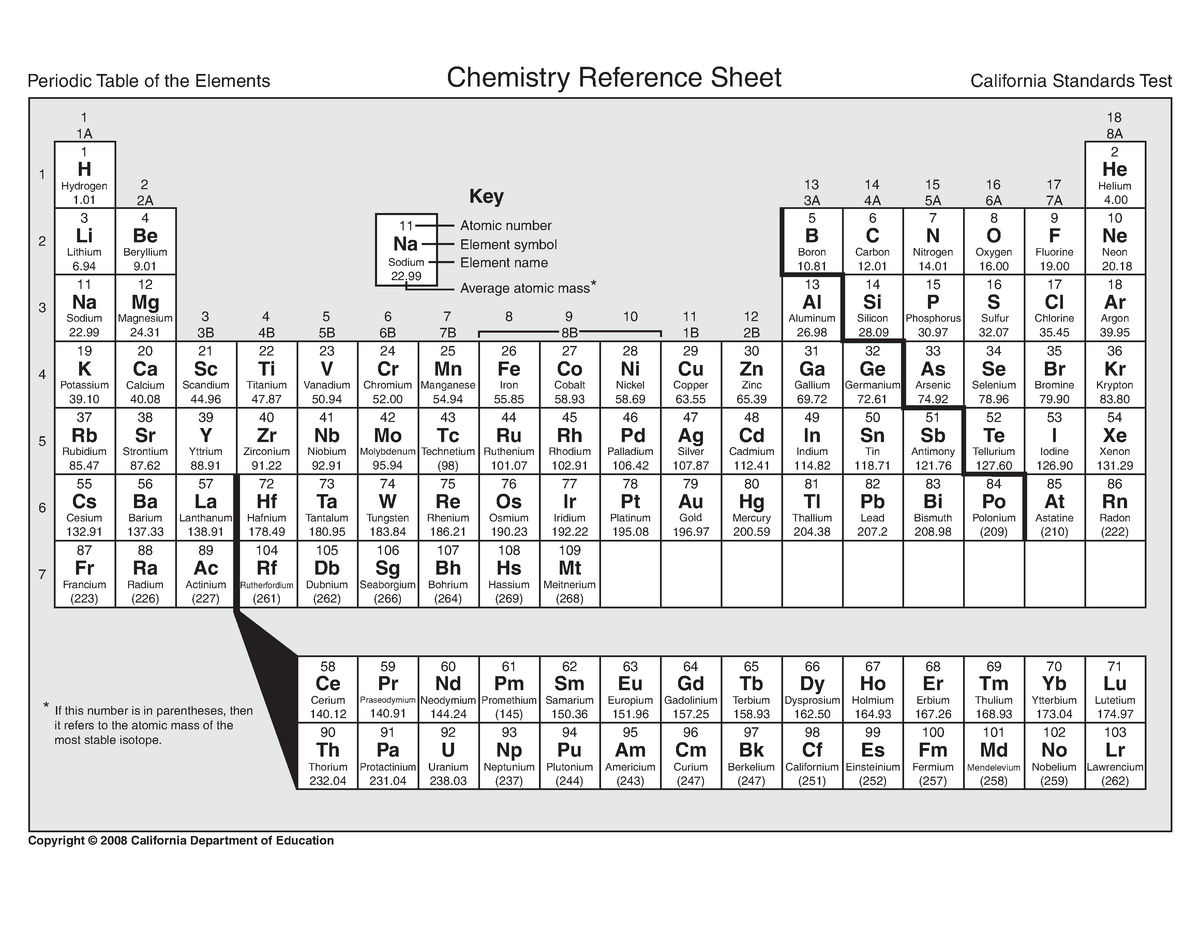
In the following centuries, the knowledge about them was expanded. The essence and importance of chemical elements was already puzzled over by the Greek philosopher Aristotle more than 2,000 years ago! Ancient man knew different elements, such as carbon or sulphur. The structure of the periodic table is the result of multi-century experiments and observations from around the world. The periodic table of elements (Mendeleev’s table)


 0 kommentar(er)
0 kommentar(er)
